The Hindu : Page 07
Syllabus : Prelims Fact
Graphene, an allotrope of carbon, exhibits exceptional properties such as strength surpassing diamond, high conductivity exceeding silver, and flexibility greater than rubber.
About Graphene:
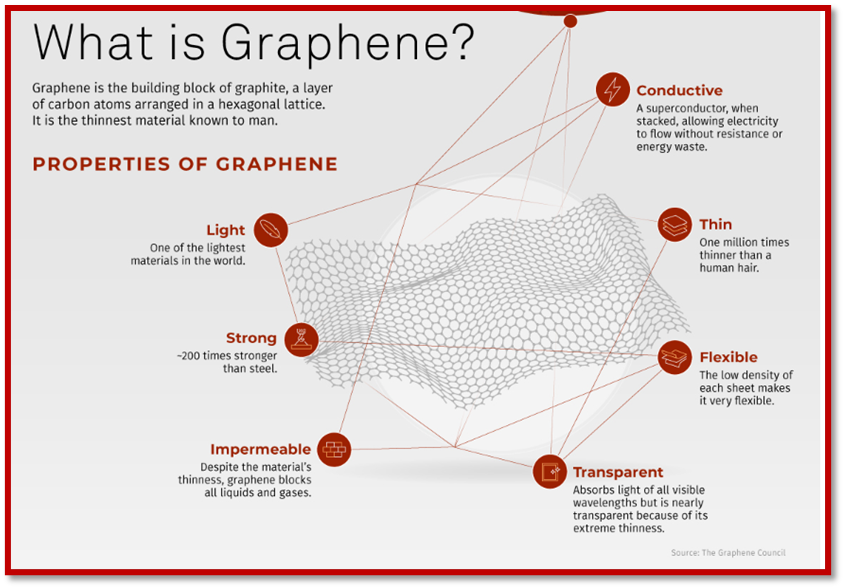
o Strength: Stronger than diamond, making it highly durable.
o Conductivity: More conductive than silver, facilitating efficient electricity and heat transfer.
o Flexibility: More elastic than rubber, allowing it to bend and stretch without damage.
o Lightweight: Lighter than aluminium, contributing to its versatility in various applications.
o Initially demonstrated by using scotch tape to peel graphite layers.
o Industrial methods like chemical vapour deposition (CVD) are used for large-scale production.
o Reinforcing materials such as car tires to improve strength and durability.
o Enhancing concrete strength by 25% while reducing carbon emissions.
o Forms a superconductor when two graphene sheets are stacked and rotated at 1.1 degrees.
o Investigated for potential applications in quantum computing and other advanced technologies.
Graphene’s unique combination of properties makes it a promising material for revolutionising various industries, from electronics